Properties of Water and the pH Scale
- Due Nov 15, 2023 at 11:59pm
- Points 25
- Questions 10
- Available after Nov 8, 2023 at 12am
- Time Limit None
- Allowed Attempts 5
Instructions
Lesson Clarity
What I will be learning.....
I will be learning about the unique properties of water, about acids, and the pH scale.
Why I will be learning this....
Life would not be possible on Earth if water didn't have its unique properties. The polarity of water, acids and bases help many of the functions of our bodies.
How I will know I learned this.....
I will be able to discuss the properties of water, how acids and bases are created, and how the pH scale functions.
WATER IS LIFE......
Water has special properties that make life possible, and allow organisms to maintain their temperature and hydration. Cells count on polar molecules, ions, to dissolve in water and diffuse into cells. Would life have begun on Earth without the special properties of WATER. Students will learn about the importance of hydrogen Bonds.
The Importance of Water For Life- water has special properties that have allowed life to develop and are critical for the function of organism and their cells. What we will learn about water.
1.The Hydrogen Bond gives water unique properties
2.Water is the universal solvent
3.Water has a high heat capacity
4.Water has a high heat of vaporization
5.Cohesion and Adhesion
6.Less dense as a solid
THE HYDROGEN BOND
Definition: The weak attraction between water molecules, due to the slight polar charges on a water molecule.
Because Oxygen is larger, stronger atom. When water is created it makes a covalent bond between two hydrogens and one Oxygen. Oxygen “hogs” the e+. In a covalent bond the electron(s) that are shared orbit both bonded atoms. However in water the e+ spends more time orbiting the far side of the Oxygen atom. This creates a slight positive end by the hydrogen atoms, because of their proton, and a slight negative end of the molecule, because the electron is mostly hanging out on the Oxygen.
.
Hydrogen bonds give water many of it’s special properties.
The hydrogen bond is strong enough that water molecules stay together, but weak enough that the bond can break and the molecules can flow past each other.
Here water collects above the rim of a glass, it’s hydrogen bonds keeping it from flowing over.
Water is the universal solvent
Water is great at dissolving polar or charged molecules. Uncharged molecules don’t dissolve well in water. It helps get polar molecules into the cell. Molecules are called hydrophillic when they have a charge themselves and are attracted to water. Hydrophillic means water loving. Hydrophobic molecules do not have a charge and are not attracted to water. They are water "hating" molecules and they do not dissolve in water. This would be molecules like oil.
Water has a high heat capacity
Water has a high SPECIFIC HEAT- the amount of energy required to raise the temperature of water by 1°- Hydrogen bonds make it harder to raise the temperature of water. Organisms have a narrow temperature range at which they can live. Organisms are made of large amounts of water. If it was easy to raise the temperature of water, organisms would die.
Water Has A High Heat of Vaporization Threshold
Hydrogen bonds make a higher threshold to go from liquid to vapor. This is important to organisms cooling themselves. Since water with hydrogen bonds holds more heat, you are less likely to dehydrate during physical activity. When it does evaporate from sweat on your skin, it carries away larger amounts of heat.
Cohesion and Adhesion
Cohesion- the property of water being attracted to other water molecules. Because water forms droplets, thousands of organisms can live in a droplet.
Adhesion- Water adheres to other objects due to its polarity. This adherence can create capillary action, and it helps trees and plants transport water up to the top of tree or plant. Capillary action is, the process of a liquid flowing in a narrow space without the assistance of, or even in opposition to, any external forces like gravity.
SOLID WATER IS LESS DENSE THAN LIQUID WATER DUE TO HYDROGEN BONDS.
Consider, would life on Earth have developed if solid water was more dense than liquid water?
pH- The acidity of things
pH is a funny symbol and not everyone agrees on its exact definition. Most scientists say that it stands for "Potential Hydrogen" and the pH of a substance tells us how may Hydrogen Ions are in the substance. Remember that an atom which has gained or lost electrons and is now charged is called an ion. Ions form Ionic bonds, with one atom keeping the electron, and both atoms attracted to each other because of their charges. Some chemical reactions leave Ions unpaired and ready to react with other atoms. Ions are very chemically reactive.
To understand pH, we should first understand some symbols. First, this symbol: (aq) means "aqueous", meaning a substance disolved in water. The symbol (l) stands for liquid. The symbol (g) stands for a gas. The symbol (s) stands for solid. When a scientist uses them in a chemical equation he or she is trying to tell the reader about the "state of matter" for the reactants and the products in that equation. Aqueous is important, because there are many substances that break down in water, and release hydrogen ions. For instance, if I were to place some Hydrochloric acid in water, it would break down into H+ ions and Cl- ions. On the periodic table hydrogen has only 1 proton, 1 electron, and no neutron. So, when the electron is taken away from Hydrogen......all you have left is a proton.
Autoionization of Water
Water has polar molecules. They have a slightly negative charge at the Oxygen end of the molecule and a slightly positive charge at the Hydrogen end of the molecule. A very, very small amount of water in any container of water, has a reaction that turns water molecules into Hydrogen Ions, H+, and Hydroxide Ions, OH-. This spontaneous reaction is called autionization, and is caused by the polar molecule of water bumping into each other just right. In any container of water, the concentration of hydrogen ions is very, very small.... 1 X10-7 H+ ions.
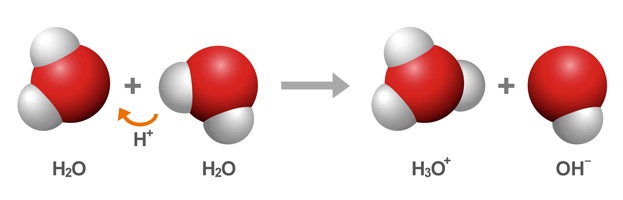
If we were to compare that to orange juice, orange juice has a greater concentration of H+ in aqueous solution.....1 x 10-4, which means it is 1000 times more acidic than water. Rather than trying to figure out long logarithmic problems, scientists have made a pH scale for us to refer to for quick comparisons. This chart shows how the logarithmic scale of pH works. Water is considered to have a neutral pH of 7. THe conentration of H+ ions in water is 1 x10-7 and translates to 7 on the pH scale. The closer to "0" the exponent is, the greater number of H+ ions or OH- ions. A pH of zero is very, very acidic. A pH of 14 is very, very basic, or alkaline- another word for a base. Each step is a multiple of 10. So if I go from 7 to 6 on the scale, my substance is 10 times more acidic. If I go three steps on the scale, 7 to 4, the substance is 1000 times more acidic....meaning it has 1000 times the concentration of Hydrogen ions.

A buffer is a solution that can resist pH change upon the addition of an acid or a base. Our blood is within normal limits, WNL, when it is between a 7.35 and a 7.45 pH. When we breath in oxygen, we use it for cellular respiration which puts back CO2 into our bloodstreams. If we do not process that out of our system well, like we are having a respiratory problem, our blood can become acidic, and if the cellular respiartion rate is too low, our blood can become too basic. Our bodies balance that need with an equilibrium reaction. If we get too acid, a chemical reaction starts to bring us back to normal. If we get too basic, a reaction happens to bring us back to normal. These are called buffering reactions that happen in our bloddstream to keep us from getting to acidic of blood, called acidosis, from carbonic acid, or too basic, called alkalosis. Acidosis can lead to shock, coma or death. Alkalosis can lead to irritability and muscle spasms.
Human cells have a pH of about 6.8, our blood about 7.4, and our stomach of 1-2. It is so acidic the lining of our stomachs is replaced every 7 to 10 days. Below is a chart for comparison of common acids and bases. A strong base can be just as destructive as a strong acid.